Segmentation of 3D HeLa Cells
HeLa-Cell-Segmentation
Segmentation, Measurement and Visualisation of Nuclear Envelope of HeLa Cells observed with Electron Microscope
Table of Contents
HeLa Cells
Citation
Data sets
Brief Description
Limitations
Running the code
Results
More input parameters
Region of Interest from 8000 x 8000 images
Input Options
Automatic cropping of multiple Regions of Interest
Visual validation of the output
Automatic cropping of Regions of Interest
Processing an entire 8,000 x 8,000 x 518 volume data set
Segmentation of Nuclear Envelope of HeLa Cells observed with Electron Microscope
This code contains an image-processing pipeline for the automatic segmentation of the nuclear envelope of HeLa cells observed through Electron Microscopy. This pipeline has been tested with a 3D stack of 300 images. The intermediate results of neighbouring slices are further combined to improve the final results. Comparison with a hand-segmented ground truth reported Jaccard similarity values between 94-98% on the central slices with a decrease towards the edges of the cell where the structure was considerably more complex. The processing is unsupervised and each 2D Slice is processed in about 5-10 seconds running on a MacBook Pro. No systematic attempt to make the code faster was made.
Citation
This work has been published in PLOS ONE (2020) and the Journal of Imaging (2021, 2019). If you find the work or the software interesting or useful, please cite as:
C Karabağ, ML Jones, CC Reyes-Aldasoro
Volumetric Semantic Instance Segmentation of the Plasma Membrane of HeLa Cells
J. Imaging 2021, 7(6), 93; https://doi.org/10.3390/jimaging7060093
C Karabağ, ML Jones, CJ Peddie, AE Weston, LM Collinson, CC Reyes-Aldasoro
Semantic segmentation of HeLa cells: An objective comparison between one traditional algorithm and four deep-learning architectures
PloS one 15 (10), e0230605
https://doi.org/10.1371/journal.pone.0230605
Cefa Karabag, Martin L. Jones, Christopher J. Peddie, Anne E. Weston, Lucy M. Collinson, and Constantino Carlos Reyes-Aldasoro,
Segmentation and Modelling the nuclear envelope of HeLa cells, J Imaging (2019), 5(9), 75
https://doi.org/10.3390/jimaging5090075
A previous version was accepted as an oral presentation in the conference Medical Image Understanding and Analysis (MIUA) 2018 (https://miua2018.soton.ac.uk)
Automated Segmentation of HeLa Nuclear Envelope from Electron Microscopy Images, in Proceedings of Medical Image Understanding and Analysis, 9-11 July 2018, Southampton, UK.
Data sets
All the data is available.
- The 8000x8000 data sets are freely available through EMPIAR: http://dx.doi.org/10.6019/EMPIAR-10094
- The cropped images at 2000x2000 data sets are available through EMPIAR: https://dx.doi.org/10.6019/EMPIAR-10478
- The 4-class ground truth (nuclear envelope, nucleus, rest of the cell, and background) is available through Zenodo: https://doi.org/10.5281/zenodo.3874949
- The 5-class ground truth (1 nuclear envelope, 2 nucleus, 3 other cells, 4 background, 5 cell) is available through Zenodo: https://zenodo.org/record/4590903
Data sets produced with this code are hosted in a parallel repository:
https://github.com/reyesaldasoro/Hela_Cell_Data
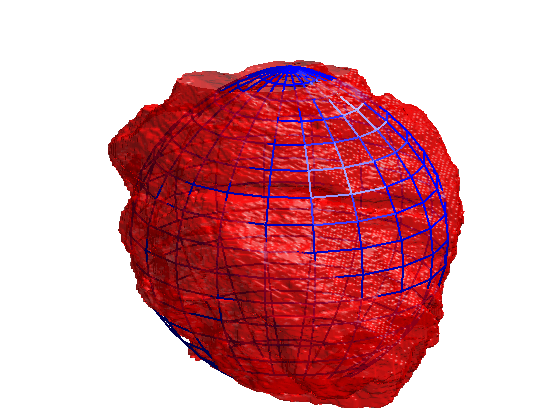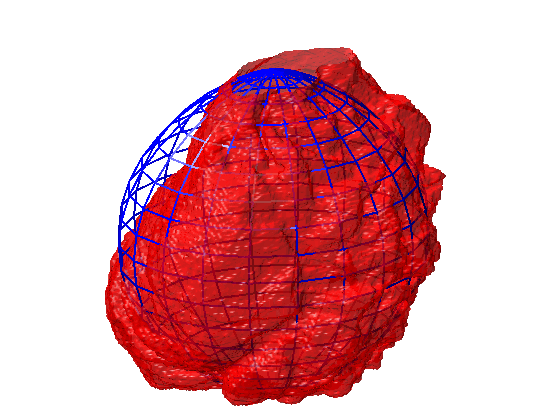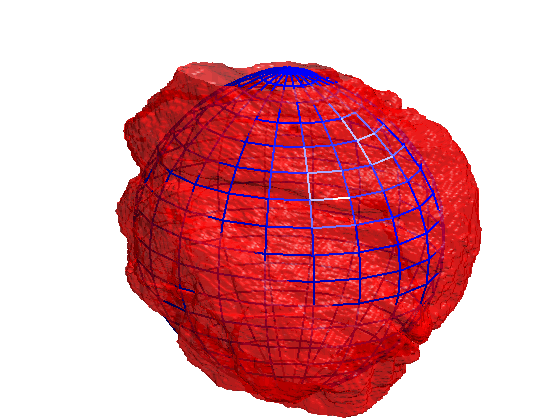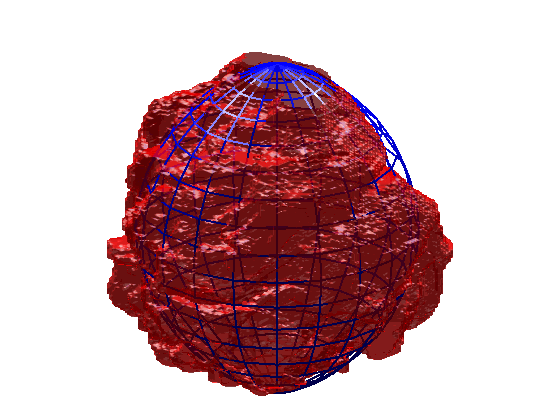
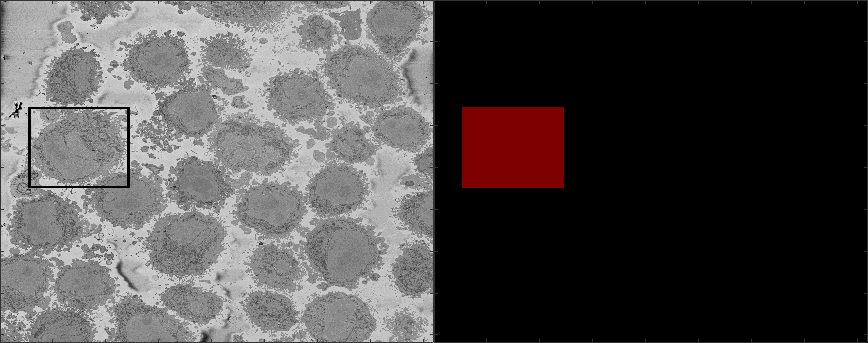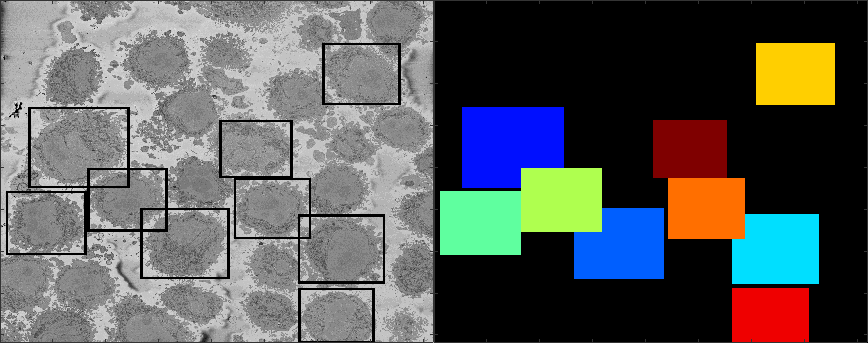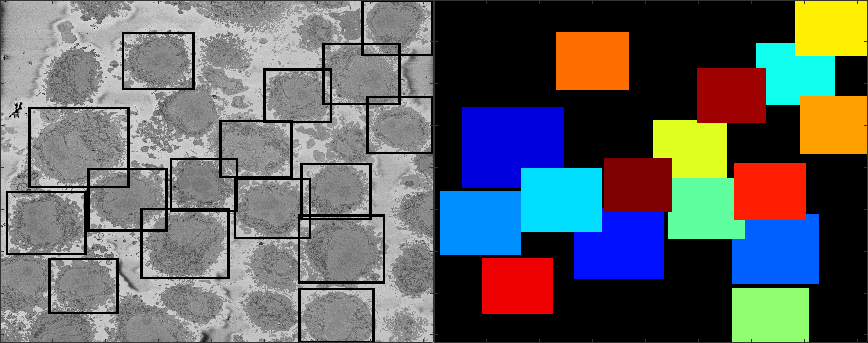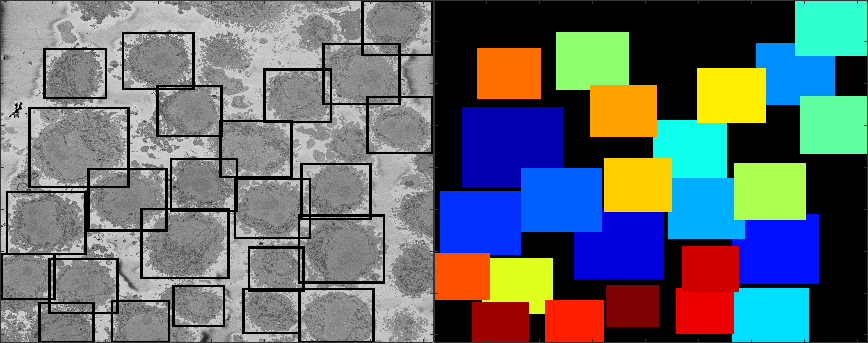
Brief description
Semantic segmentation of (a) Nuclear envelope, and (b) cell from its neighbours as observed with Electron Microscopy in 3D. The methodology consists of several image-processing steps: low-pass filtering, edge detection and determination of super-pixels, distance transforms and delineation of the nuclear and cell membranes.Limitations
The algorithm relies on a background that is brighter than the cells and their structures as can be seen in the figures of this page.
The algorithm to segment the nuclear envelope assumes the following: there is a single HeLa cell of interest, the centre of the cell is located at centre of a 3D stack of images, the nuclear envelope is darker than the nuclei or its surroundings, the background is brighter than any cellular structure.
The algorithm will segment regions of interest to ensure that there is a single HeLa cell of interest (there may be more surrounding the cell) in each cropped region..
Running the code for a single 2,000 x 2,000 x 300 voxel region of interest with one cell
(If you want to start with the 8,000 x 8,000 x 518 voxel data set, skip to Processing an entire 8,000 x 8,000 x 518 volume data set )
Assuming your image is a tiff file called 'Hela.tiff' you can pass this directly to the function segmentNucleiHelaEM as an input argument and returns the segmentation of the nuclei with 1 and the rest with 0. The background is segmented with segmentBackgroundHelaEM and similarly returns a matrix with 1 for background and 0 for the rest.
Hela0 = imread('Hela.tiff');
Hela = double(Hela0(:,:,1));
Hela_background = segmentBackgroundHelaEM(Hela);
Hela_nuclei = segmentNucleiHelaEM(Hela);
To process a stack of images, you can use the extension of the previous function through segmentNucleiHelaEM_3D. The input argument can be either:
- a folder where the stack of images (these have to be tiff images, will analyse if there are other extension later on), one image per slice of the data,
- a multidimensional TIFF file,
- a three dimensional Matlab matrix.
The outputs of this function are 2 matrices Hela_nuclei and Hela_background which will be three dimensional with as many levels as slices of the original data.
MEMORY: these functions load all the slices into a single matrix in Matlab. This has been tested with 300 slices of 2,000 x 2,000 pixels on a computer with 32GB RAM memory. Computers with less memory may have problems. If you have less memory available, You can run a loop and segment per slice as described in one of the chapters. This was tested on a computer with 8GB RAM.
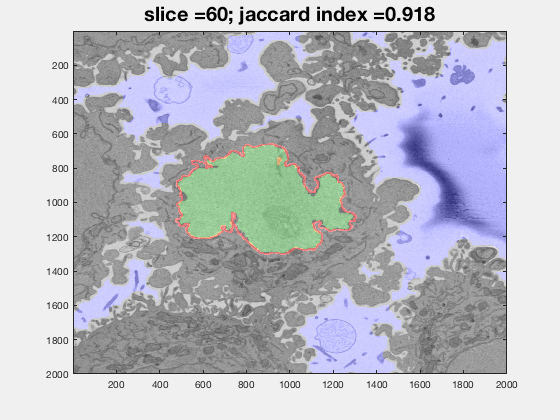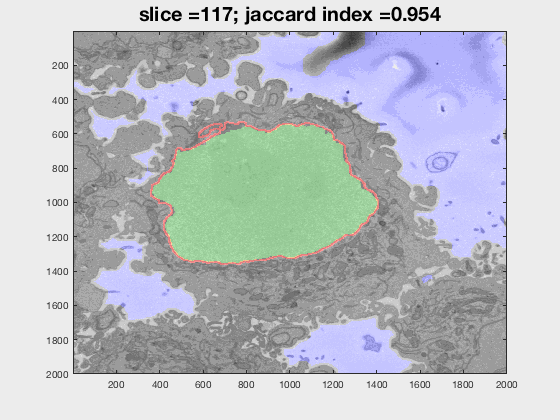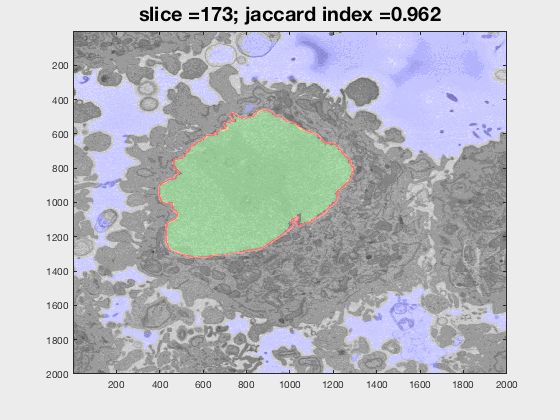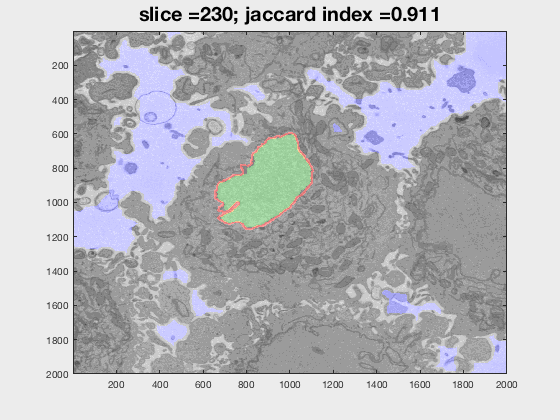
Results
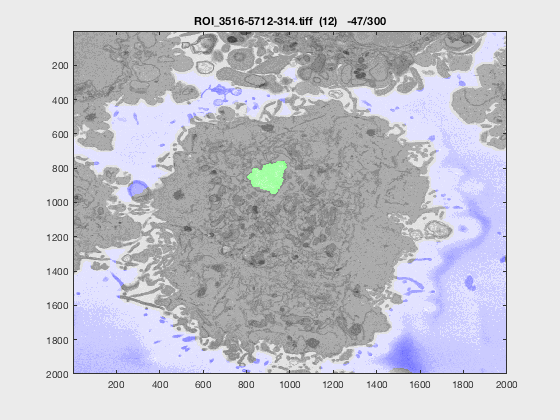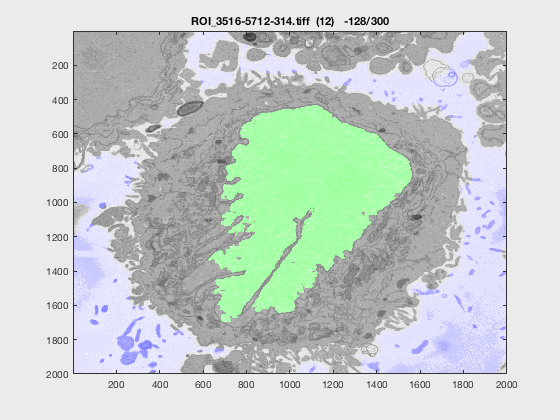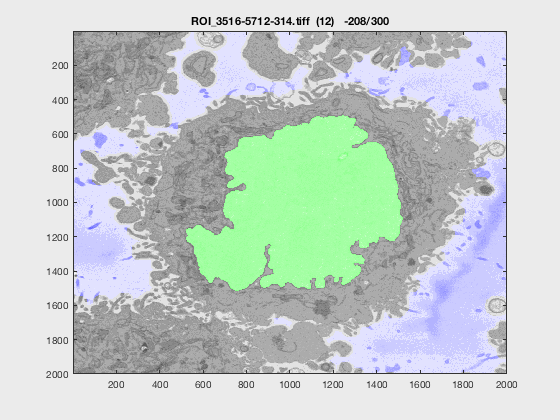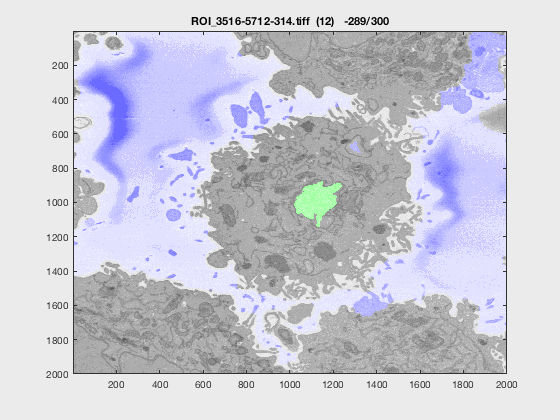
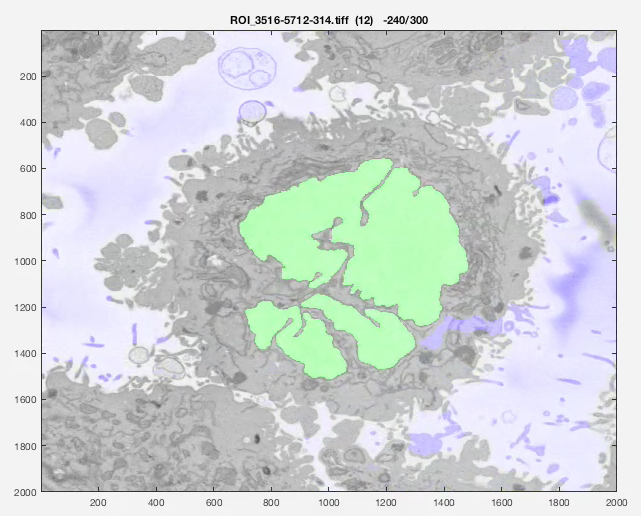
The following animation shows a multi-slice segmentation where the segmented background is shaded in purple, the segmented nuclei is shaded in green, the ground truth is a red line.
More input parameters
The code can receive 2 more parameters, one if you want to change the standard deviation of the Canny algorithm, and a previous segmentation.
nucleiHela = segmentNucleiHelaEM(Hela,previousSegmentation,cannyStdValue)
This other parameter is useful when you are processing a large number of slices, you can segment the central slice and use that as a parameter for the slices above and below. When the shape becomes irregular, this allows the algorithm to select more than one region.
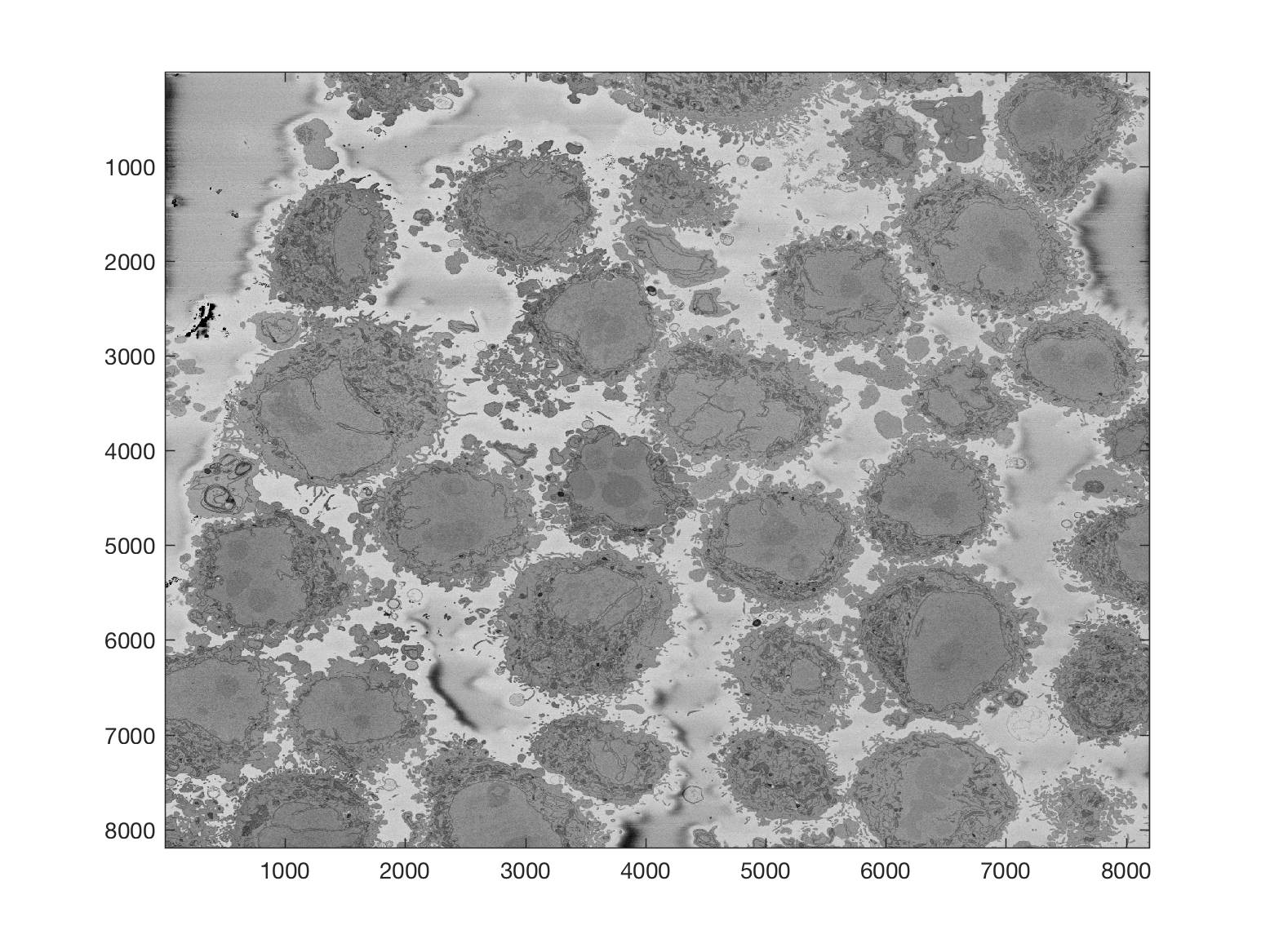
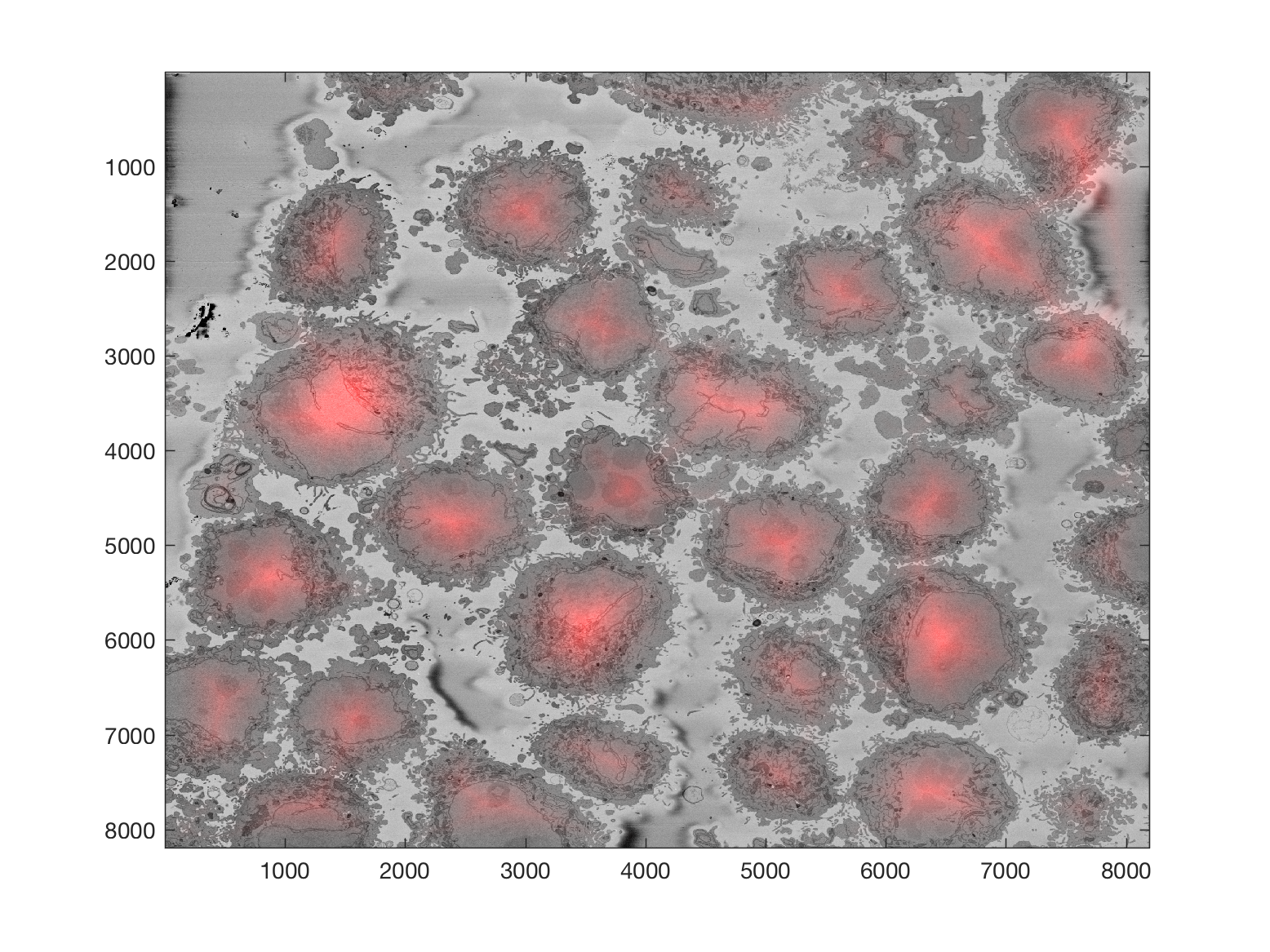
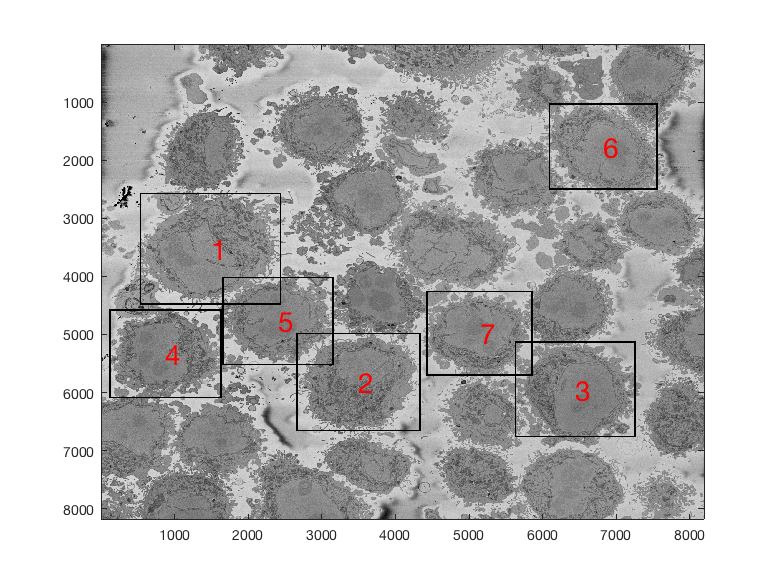
Region of Interest from 8000 x 8000 images
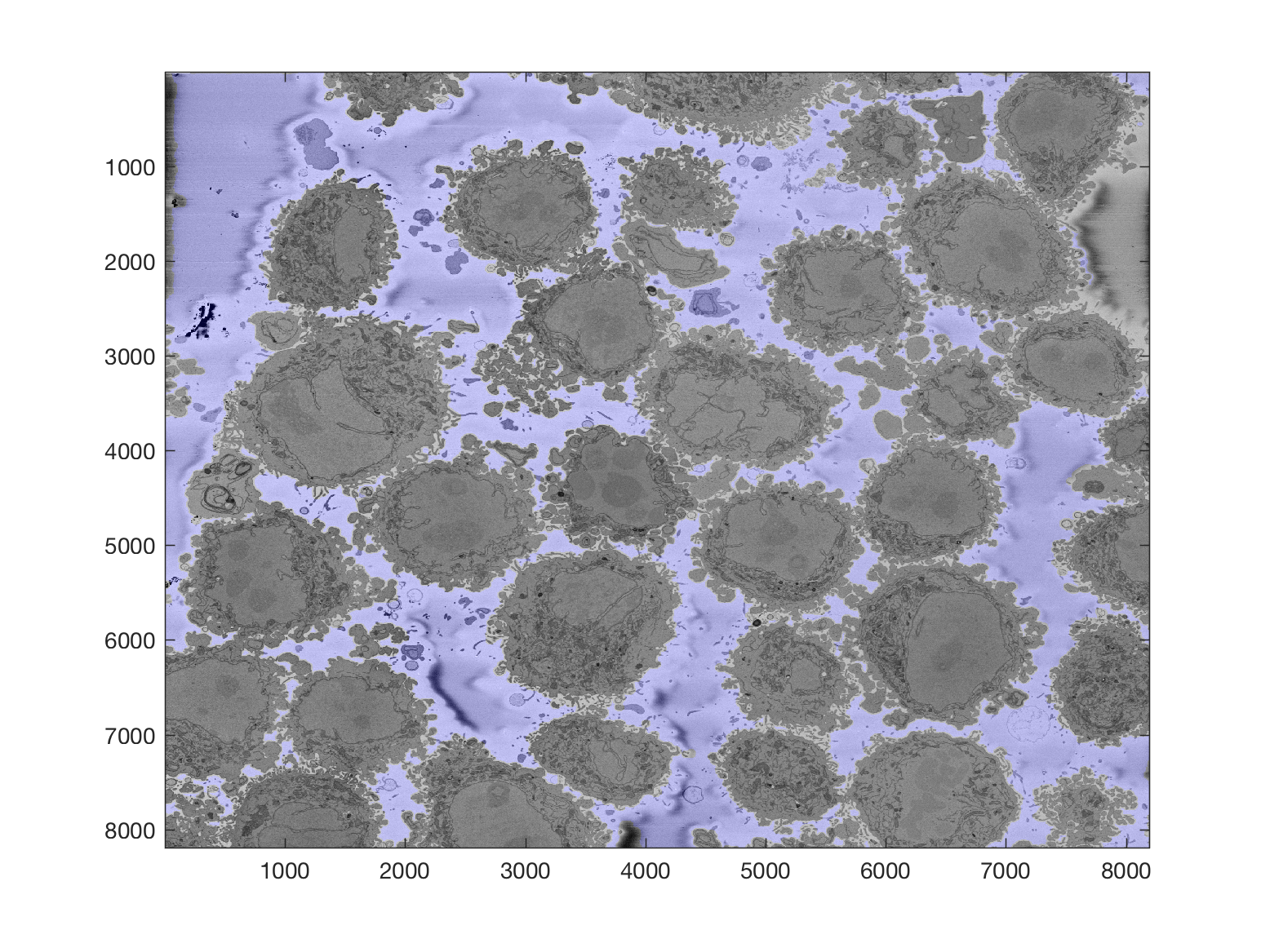
The above images have been manually cropped from a larger image by detecting the central point of the cells, and then selecting 300 slices and 2000 x 2000 pixels with the point as the centre. Whilst this is not too difficult, time consuming or error-prone, the detection of the background allows to detect automatically the majority of cells from one single plane.
Starting with one single 8000 x 8000 plane like this:
The background can be segmented as previously described to obtain this
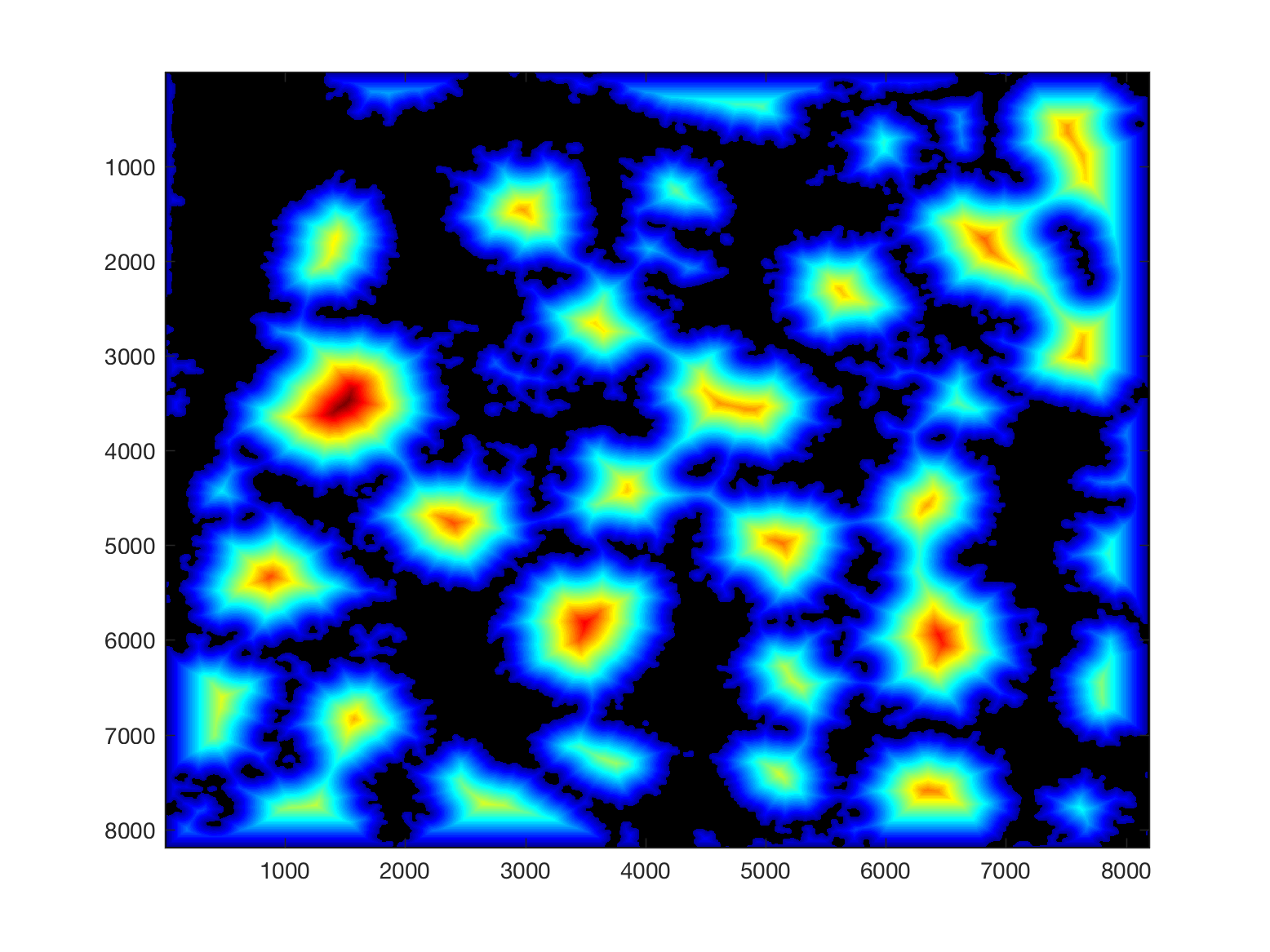
Then, from this background, it is easy to calculate a distance transform:
That distance transform is related to the cells in the image in terms of their size, and how far away they are from the background.
Thus, provided that there is some background in the plane (and that would be the main limitation of the algorithm), the cells can be detected by finding peaks on the distance, selecting a region of interest based on the height of that peak, and then proceeding to the following peak. It is important to proceed iteratively as the region of each cell may contain several "peaks", and each iteration removes all the peaks from that region.
The code runs like this
IndividualHelaLabels = detectNumberOfCells(Hela);
The segmentation will produce automatically an image for validation purposes
IndividualHelaLabels = detectNumberOfCells(hela,7);
The algorithm can stop in 2 ways, one is to request a specific number of cells, say you are only interested in the 10 largest cells (second argument to the function), or until no more cells are detected (only one argument is passed, the image itself). An assumption for this last condition is that cells will only be considered if their associated distance peaks are within a limit, currently 50% of the height of the top peak.
The output of the function is one 3D matrix with one level per cell detected. To visualise any given cell, you can just use one level of the 3D Matrix like this:
imagesc(Hela.*(IndividualHelaLabels(:,:,1))) imagesc(Hela.*(IndividualHelaLabels(:,:,2)))
Further analysis can consider distance between cells, cells that are in contact with the edge of the image, size of the cells, etc.
Input Options
There are three options to select the image to be segmented:
(1) Read an image (with "imread") and pass the variable with the 2D matrix, (2) Pass the name of the image as an argument to the function, the function will read automatically that image, (3) Pass the name of the folder where the image is located, the function will read the whole directory inside the folder, will select the middle slice and will read automatically.
>> IndividualHelaLabels = detectNumberOfCells(Hela,10);
>> IndividualHelaLabels = detectNumberOfCells('DataWholeSlice/Prefix_3VBSED_roi_00_slice_0249.tif',10);
>> IndividualHelaLabels = detectNumberOfCells('DataWholeSlice',10);
Processing an entire 8,000 x 8,000 x 518 volume data set
The number of cells to be identified can be pre-defined per slice and then the process is repeated for a number of slices of the 3D stack. This is processed like this
[final_coords,final_centroid,final_cells,final_dist] = detectNumberOfCells_3D(baseDir,20,1);
baseDir is a link to the folder that contains the 518 images, e.g.
baseDir = 'C:\Users\sbbk034\Documents\Acad\Crick\Hela8000_tiff\';
The selected number of cells to be detected per slice in this case is 20 and the final 1 is used to display the output. As the slices are processed, each with 20 cells, which are not always the same in each slice, there will be more cells detected. In this case, the algorithm has detected a total of 30 cells.
The function returns
>> whos Name Size Bytes Class Attributes baseDir 1x52 104 char final_cells 30x26 6240 double final_centroid 30x5 1200 double final_coords 30x6 1440 double final_dist 30x26 6240 double >>
Important among these ones are:
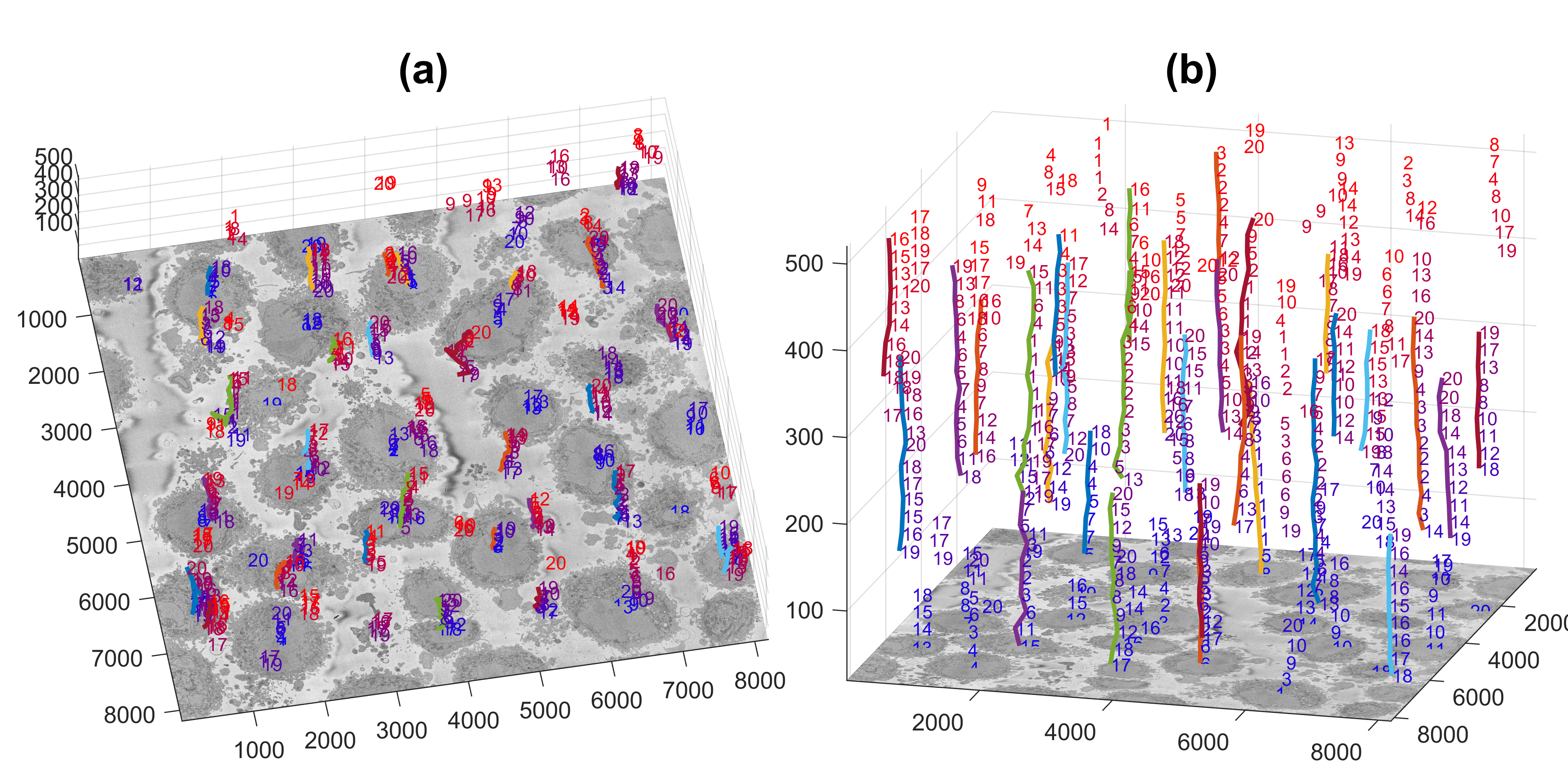
- the centroids of each cell, [row, column, z-stack position, base, top] e.g.
1280 4303 121 1 221 5825 2002 101 1 181 7312 3770 121 21 221 ...
- the coordinates to be cropped of each cell, [init row, fin row, init column fin column, init z fin z] e.g.
1 2000 665 2664 1 300 5005 7004 3739 5738 1 300 280 2279 3303 5302 1 300 ...
The centroids of the cells are linked vertically to identify which centroids correspond to the same cell. The figure below illustrates the centroids at every 20 slices (i.e. 26 slices were analysed)
The next step is to crop these cells into their own individual regions of interest so that the become a single instance of a cell. This is done with the command generate_ROI_Hela, which is used in the following way:
listFolders = generate_ROI_Hela (baseDir,final_coords,final_centroid);
The output is one folder with 300 slices each 2,000 x 2,000 pixels wide.
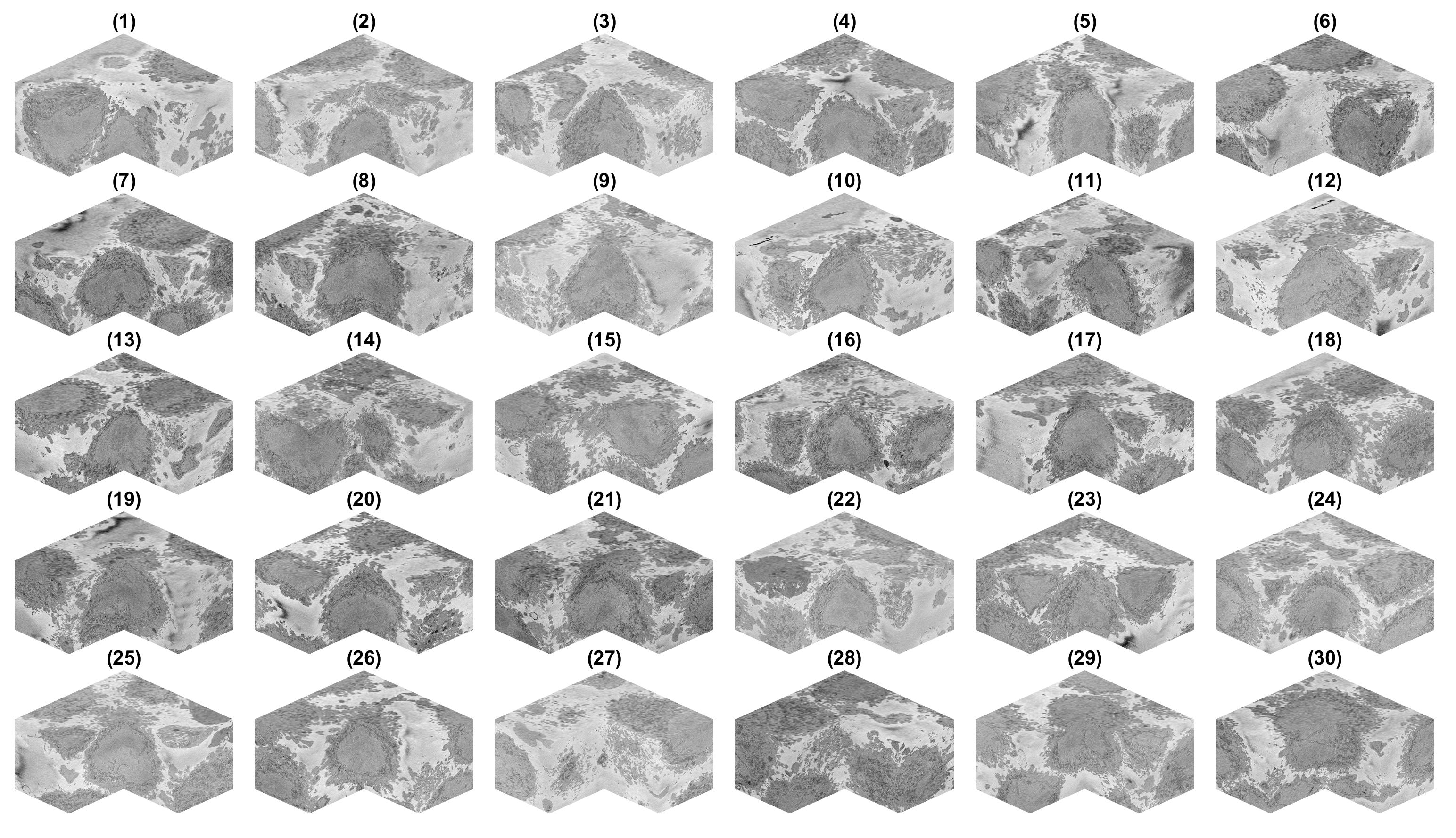
There is a decision to process all the ROIs, there may be cells that are close to the top or bottom and thus would have only part of a cell, and similarly, the centroids may have been close to the edge of the volume. It is possible to visualise these cells by displaying each volume. It is more practical to remove a corner to see how well located is each cell:
It can be seen that there are some cells that are either low and part of the cells is not within the field of view (1,2) and some others that are rather high (29,30) and part of the cell is again outside the field of view. In addition, there are others that, due to their proximity to the edges of the volume are not centred but rather to a side (6,14). Observing the variable final_centroid is possible to determine how far away (i.e. absolute distance) are the centroids from the edges. If we use a margin of 500 pixels, we can observe that the centroids of following cells are less that the margin away in at least one dimension:
6 0 417 0 14 489 746 0 15 0 432 0 27 455 489 0 28 588 465 97
There are several complications if the cell is not well centred: (a) there may be several cells and it may be that two cells could be equally valid as an instance of the cell and (b) the algorithm assumes that the cell is centred for the selection of regions.
In addition, with respect to the top and bottom edges the following volumes are close to the edges:
1 0 0 81 2 0 0 81 28 588 465 97 29 0 0 97 30 0 0 77
The situation of cells that are close to the top and bottom are slightly different, here the problem is that the segmentation will not be of a complete cell but only part of it, so the segmentation may appear as a hat when it is in the lower section of the volume or a bowl if it is in the upper section. If metrics like volume are to be obtained, then these partial sections are not to be used. However, if what is of interest is an observation, rather than a quantification, then it is not a problem.
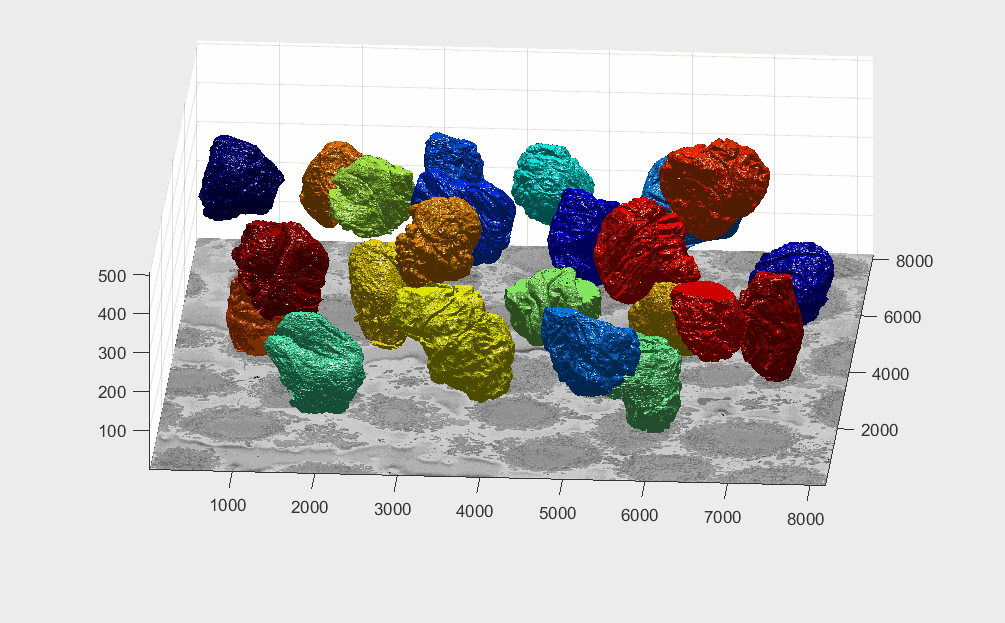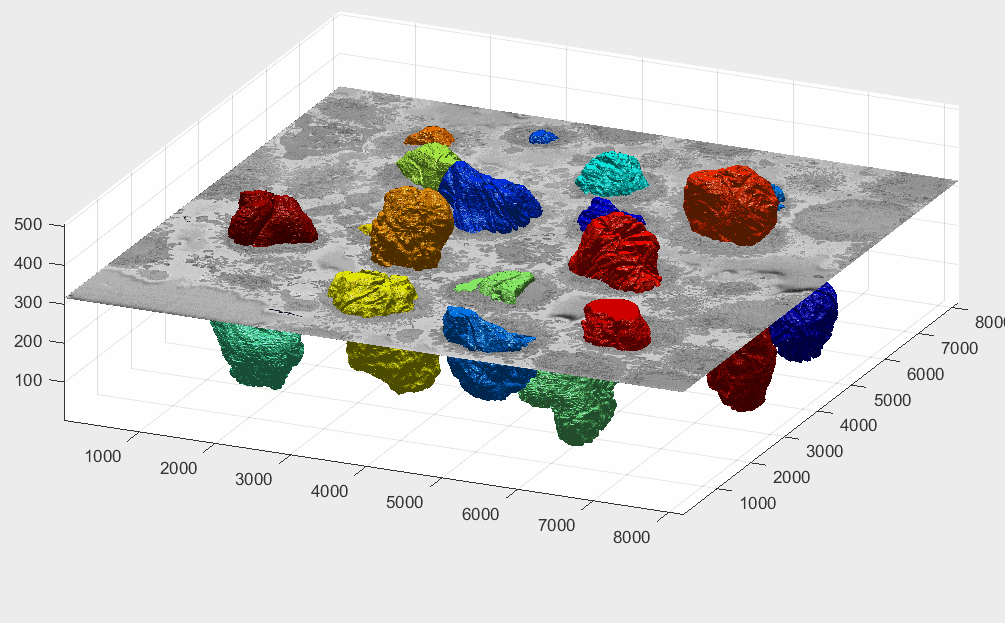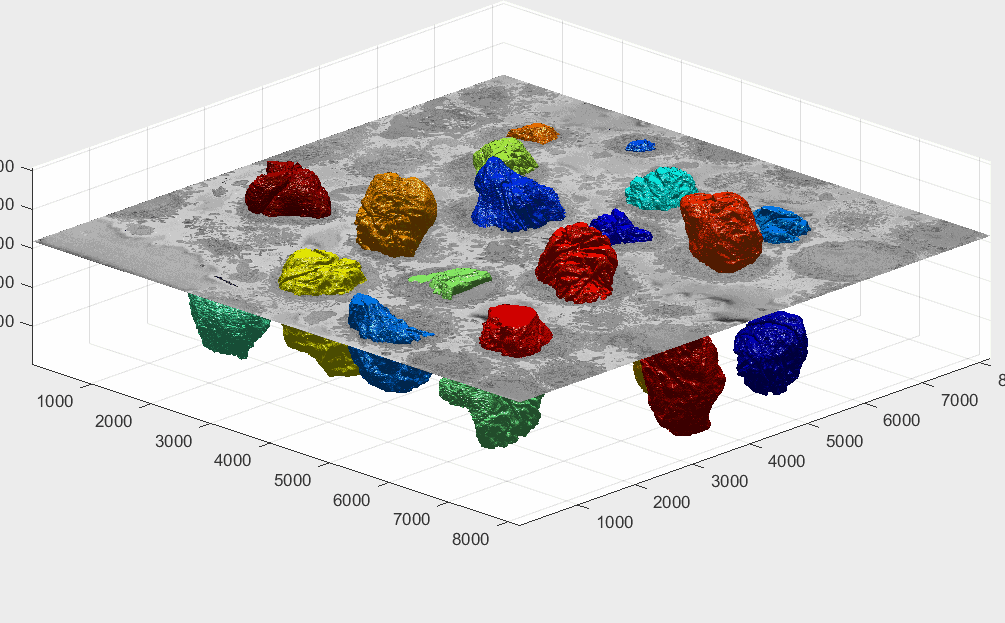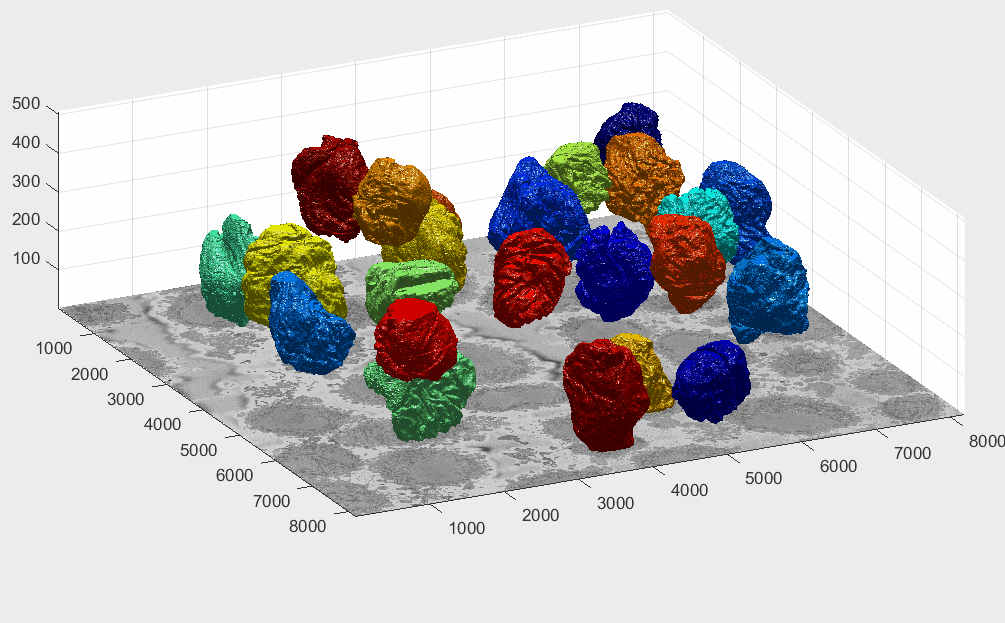
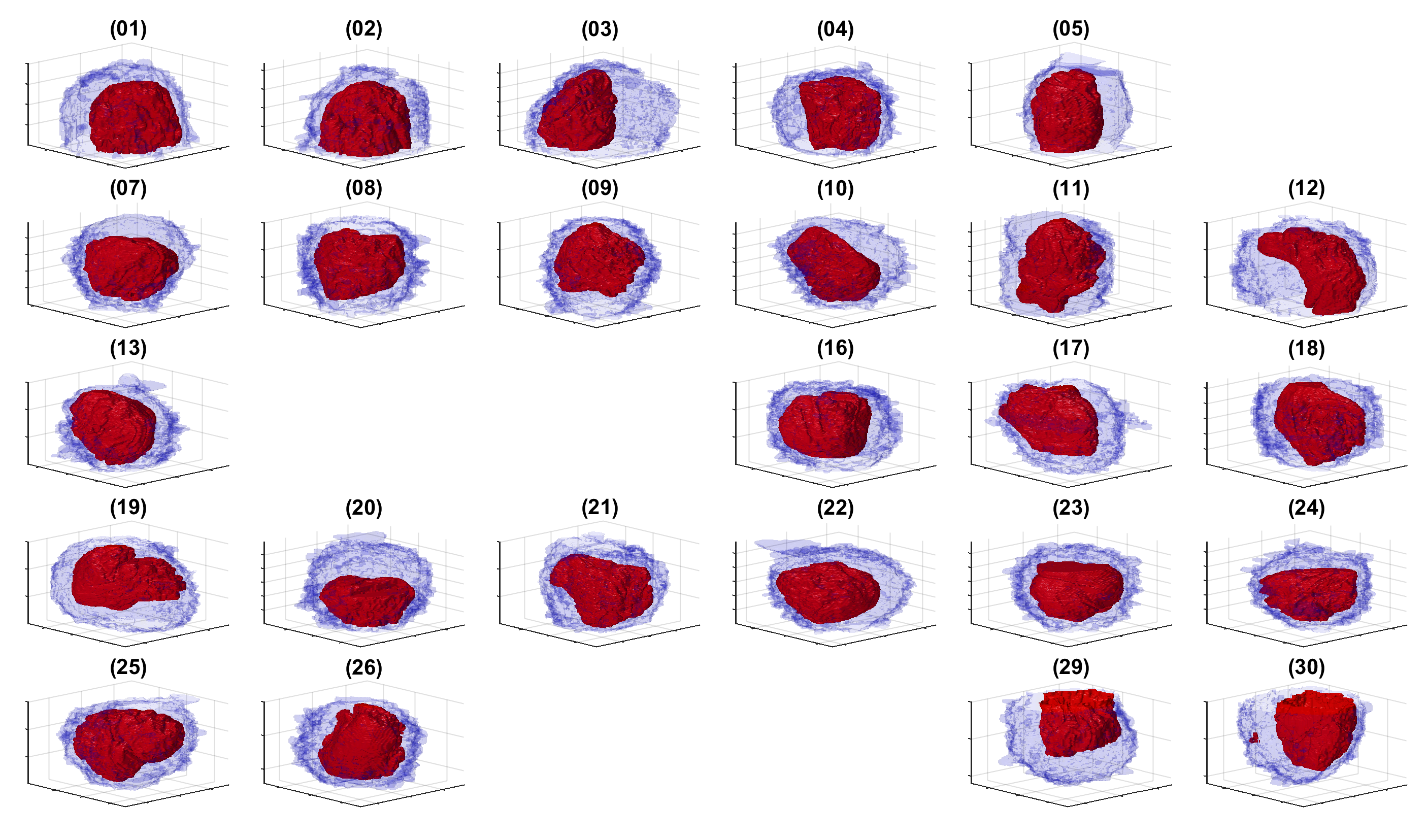
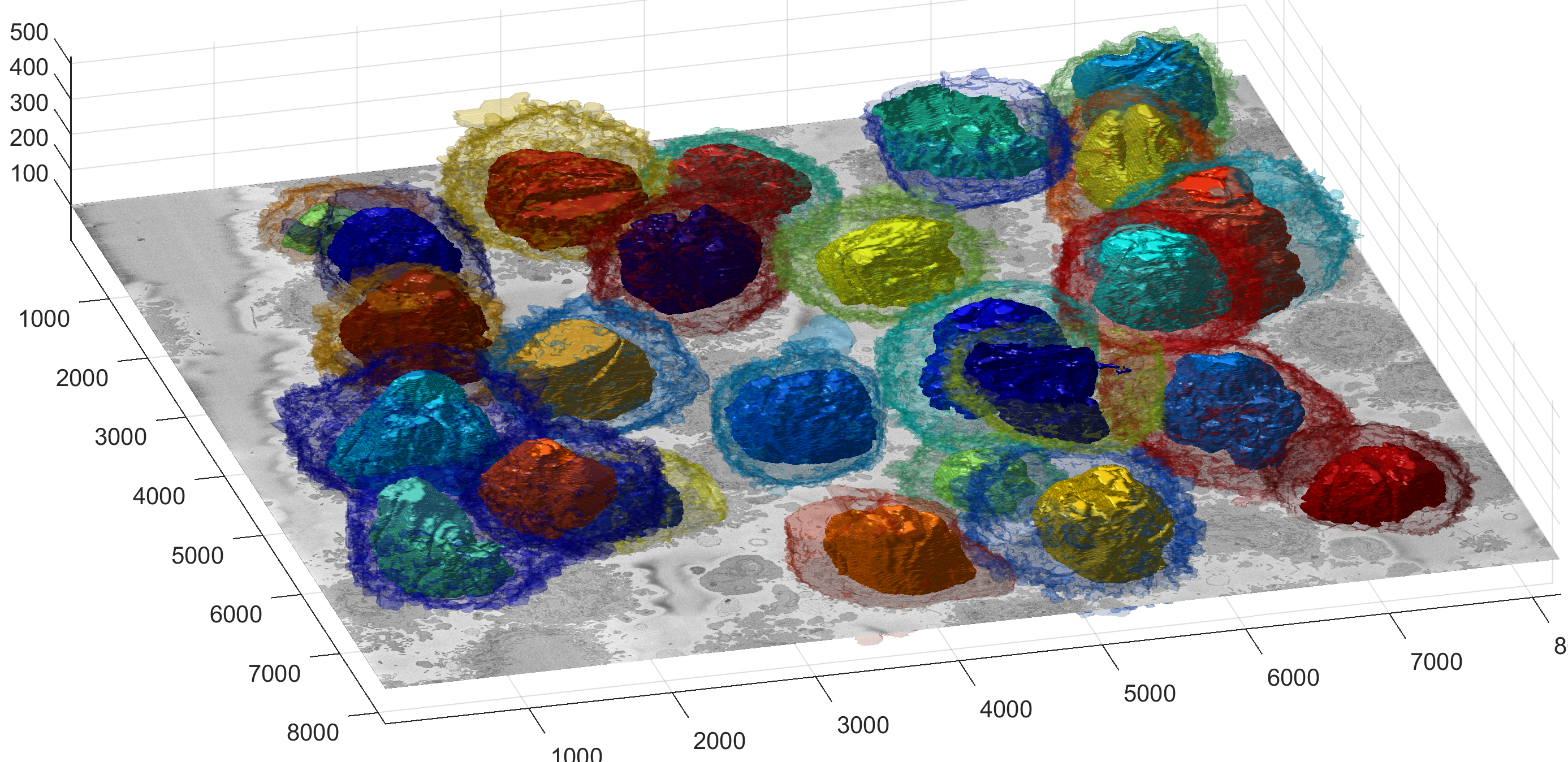
The segmentation of the cells and nuclei is shown below for 25 of the 30 cells. Cells 6, 14, 15, 27, 28 were not further processed. The cells are placed in the same location as the ROIs above to help visualisation.
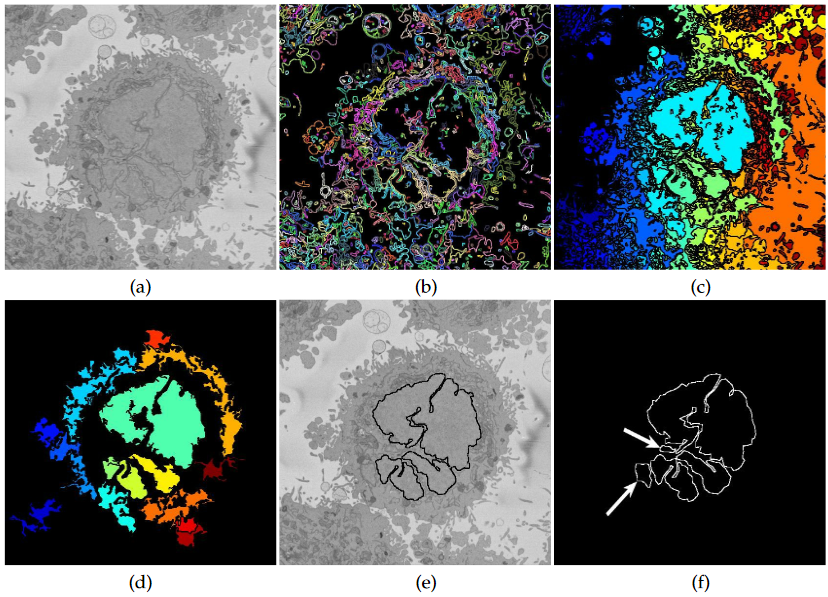
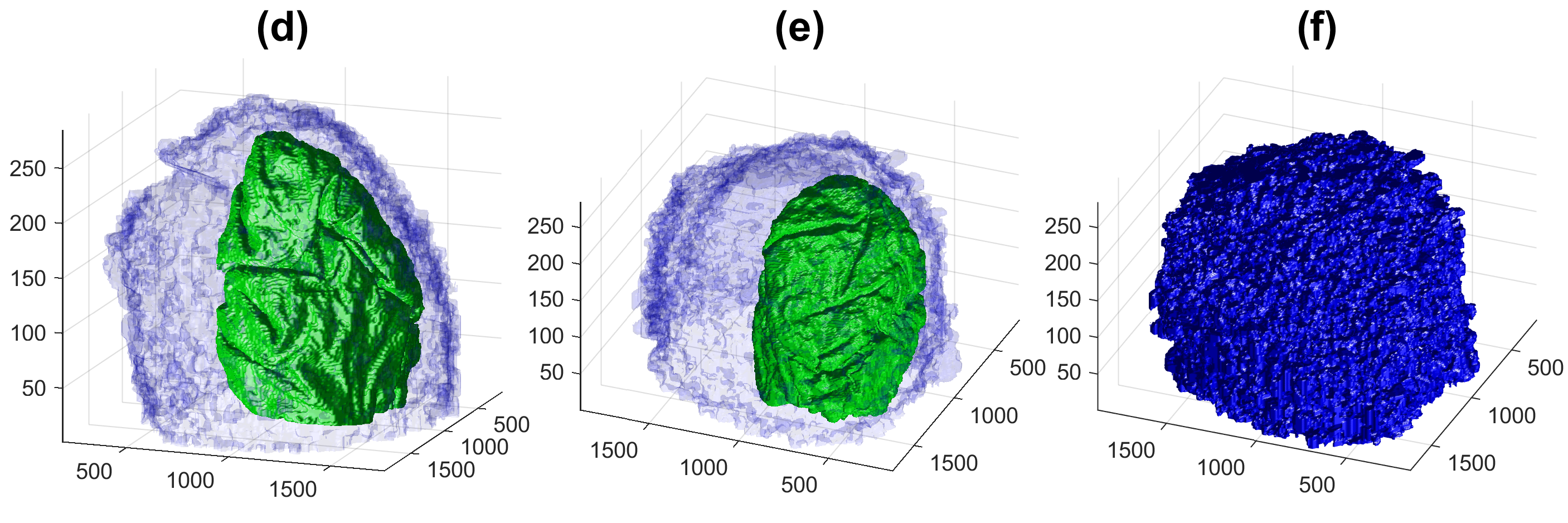
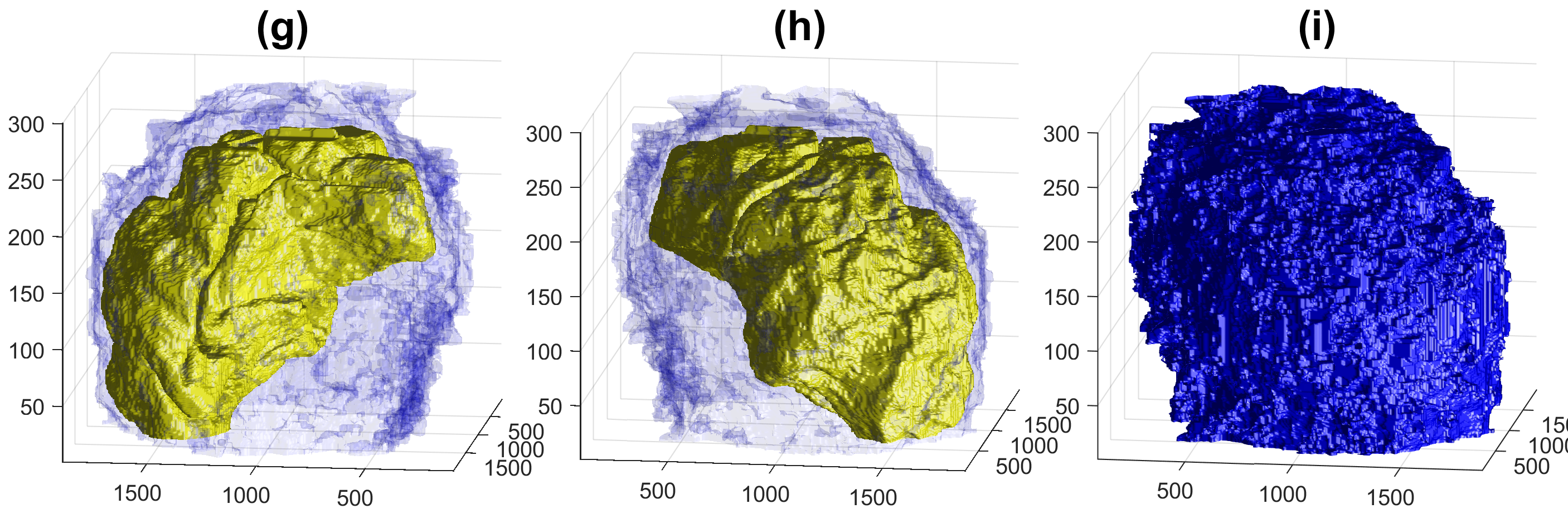
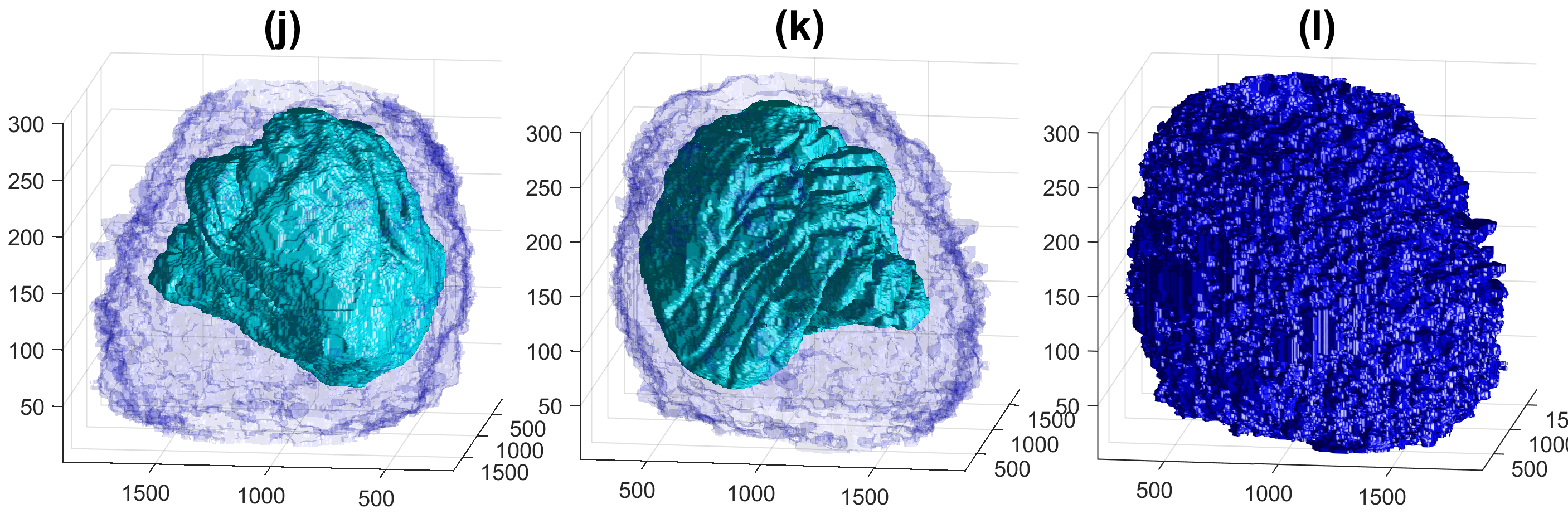
The segmentation of the nuclear envelope follows these steps: (a) low-pass filtering, (b) Edges detection, the edges were further dilated to connect those edges that may belong to the nuclear envelope (NE) but were disjoint due to the variations of the intensity of the envelope itself. (c) Superpixels were generated by removing dilated edges. (d) Small superpixels and those in contact with the image boundary were discarded and the remaining superpixels were smoothed and filled, before discarding those by size that did not belong to the nucleus. (e) Final segmentation of the NE overlaid (thick black line) on the filtered image. (f) Ground truth (GT) manual segmentation of the same image. Regions not selected by the algorithm because of their sizes are highlighted with arrows. Notice that both the algorithm and GT detected disjoint regions.
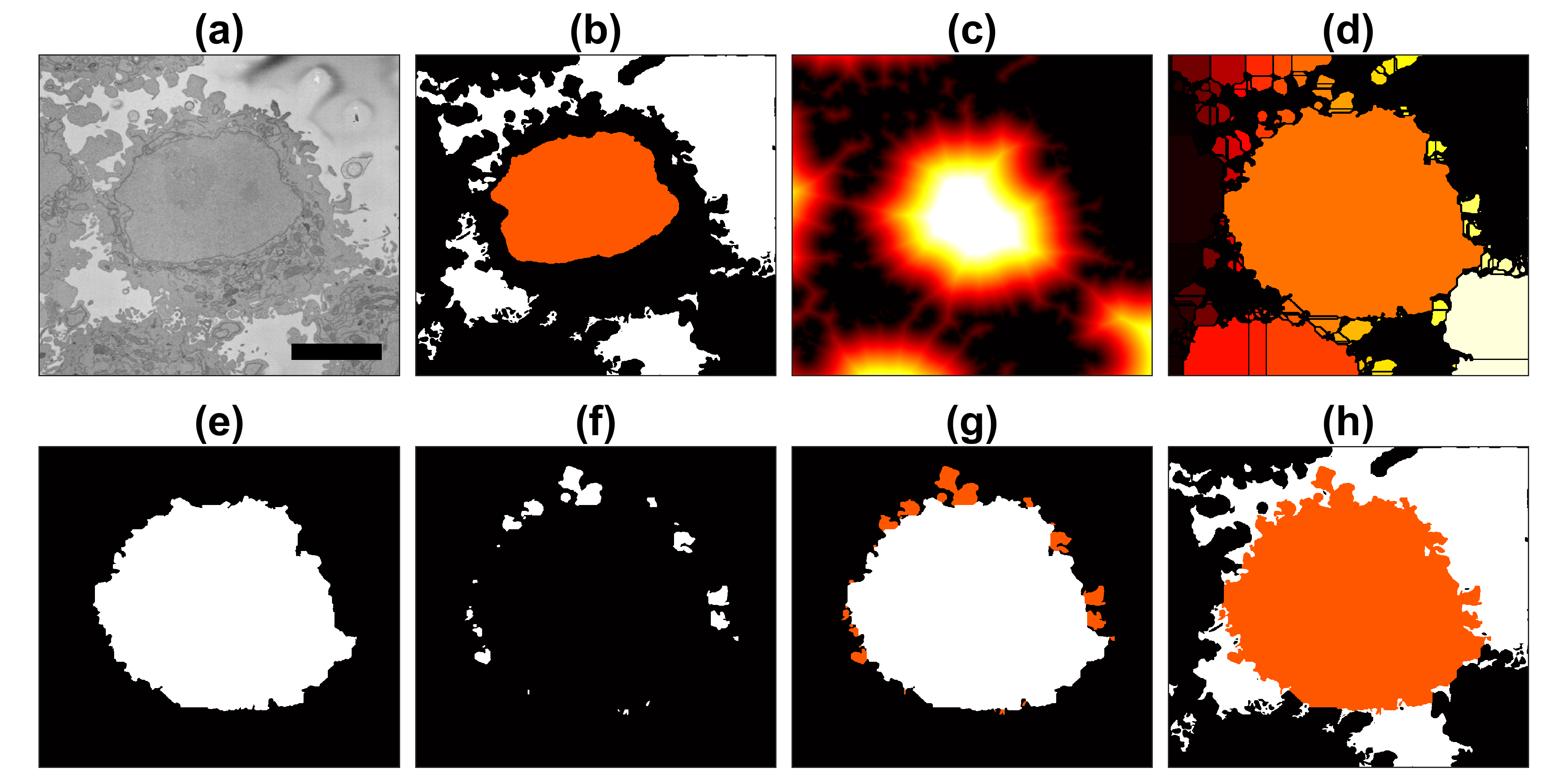
The segmentation of one cell from its neighbours follows these steps: distance transformation from the background which grows around regions with cells and since there will be one larger hill at the centre. The distance transformation is then segmented with a watershed algorithm. The central region is morphologically opened with large structural elements to remove protruding artefactual regions. This provides a fairly round cell that does not include the natural protrusions. Thus, regions that are contiguous to this central region and surrounded by background are identified and merged with the cell.
To process a 3D stack the commands are the following:[Hela_nuclei,Hela_background] = segmentNucleiHelaEM_3D(listFolders{k});
[Hela_cell] = segmentCellHelaEM_3D(Hela_nuclei,Hela_background,[]);
When the cells are not vertically centred as those previously mentioned, it is possible to add an optional parameter given by the previously calculated centroid:
[Hela_nuclei,Hela_background] = segmentNucleiHelaEM_3D(listFolders{k},(final_centroid(30,3)-final_coords(30,5)));
[Hela_cell] = segmentCellHelaEM_3D(Hela_nuclei,Hela_background,[],(final_centroid(30,3)-final_coords(30,5)));
The second and fourth parameters (final_centroid(30,3)-final_coords(30,5)) indicate that the cell is not vertically centred and should be processed from that location.
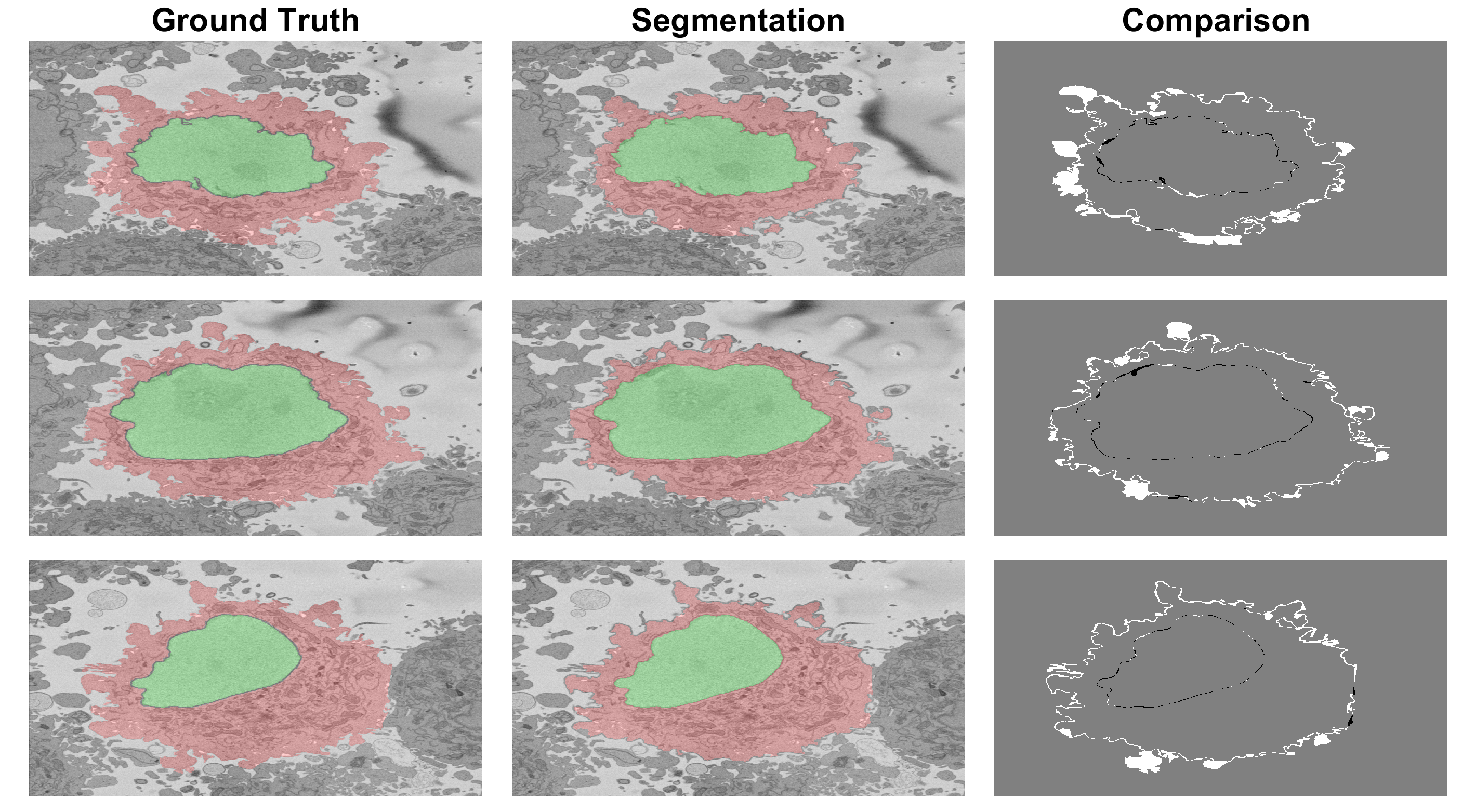
The results are quite good when the cell is surrounded by background and cautious when there are cells close to each other. The figure below shows a comparison between ground truth and segmentation. Left column shows GT (green for nucleus) and cell (red). Centre column shows segmentation. Right shows comparison between GT and the results with FP in white, FN in black and both TP and TN in gray.
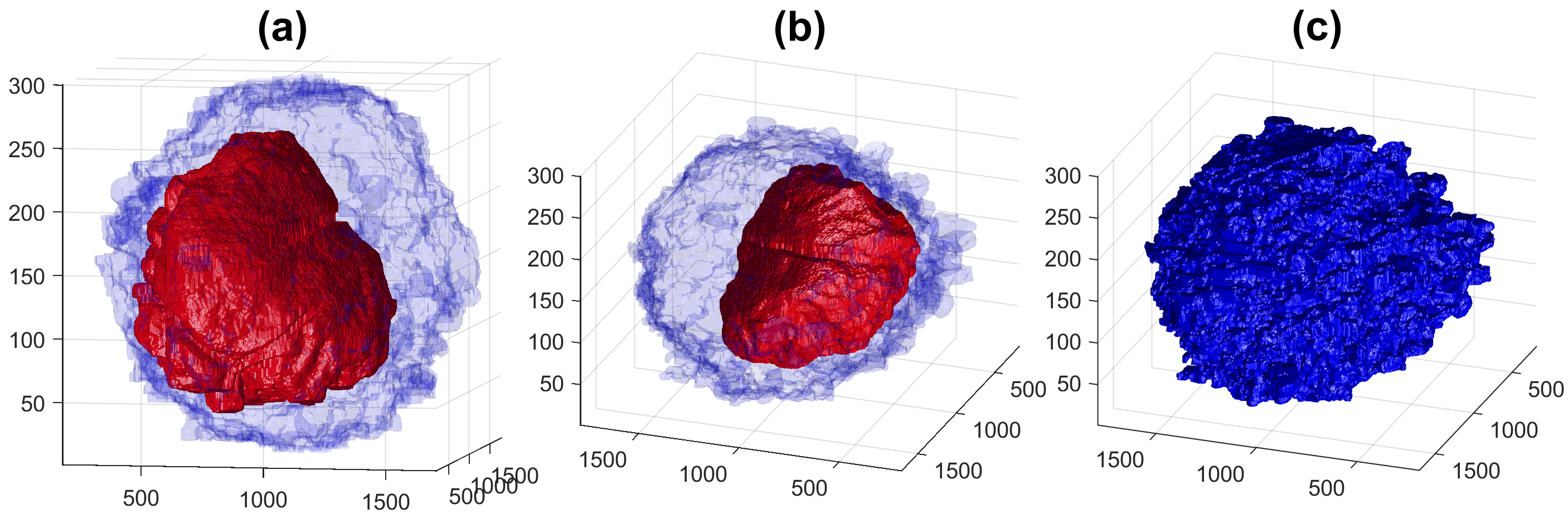
Four examples of volumetric reconstruction of the nuclear envelope and the cellmembrane of HeLa cells. In all cases, each row corresponds to a single cell observed from different view points. Left and centre columns show the cell membrane with transparency. Right column the cell membrane without transparency from the same view point as centre column.
Illustration of segmentation of 23 cells and nuclear envelopes. The cells were segmentedfrom a 8192×8192×518 voxel region. Slice number 100 out of 518 is displayed for context. Nuclei are solid and cell membranes are transparent. Colours have been assigned randomly for visualisation purposes.
Illustration of the nuclei only.
Cite As
Constantino Carlos Reyes-Aldasoro (2024). Segmentation of 3D HeLa Cells (https://github.com/reyesaldasoro/HeLa-Cell-Segmentation/releases/tag/v1.0.0), GitHub. Retrieved .
Karabağ, Cefa, et al. “Segmentation and Modelling of the Nuclear Envelope of HeLa Cells Imaged with Serial Block Face Scanning Electron Microscopy.” Journal of Imaging, vol. 5, no. 9, MDPI AG, Sept. 2019, p. 75, doi:10.3390/jimaging5090075.
Karabağ, Cefa, et al. “Semantic Segmentation of HeLa Cells: An Objective Comparison between One Traditional Algorithm and Four Deep-Learning Architectures.” PLOS ONE, edited by Yuanquan Wang, vol. 15, no. 10, Public Library of Science (PLoS), Oct. 2020, p. e0230605, doi:10.1371/journal.pone.0230605.
Karabağ, Cefa, et al. “Volumetric Semantic Instance Segmentation of the Plasma Membrane of HeLa Cells.” Journal of Imaging, vol. 7, no. 6, MDPI AG, June 2021, p. 93, doi:10.3390/jimaging7060093.
MATLAB Release Compatibility
Platform Compatibility
Windows macOS LinuxTags
Community Treasure Hunt
Find the treasures in MATLAB Central and discover how the community can help you!
Start Hunting!Discover Live Editor
Create scripts with code, output, and formatted text in a single executable document.
Code
MatlabFigs
| Version | Published | Release Notes | |
|---|---|---|---|
| 1.0.0 |