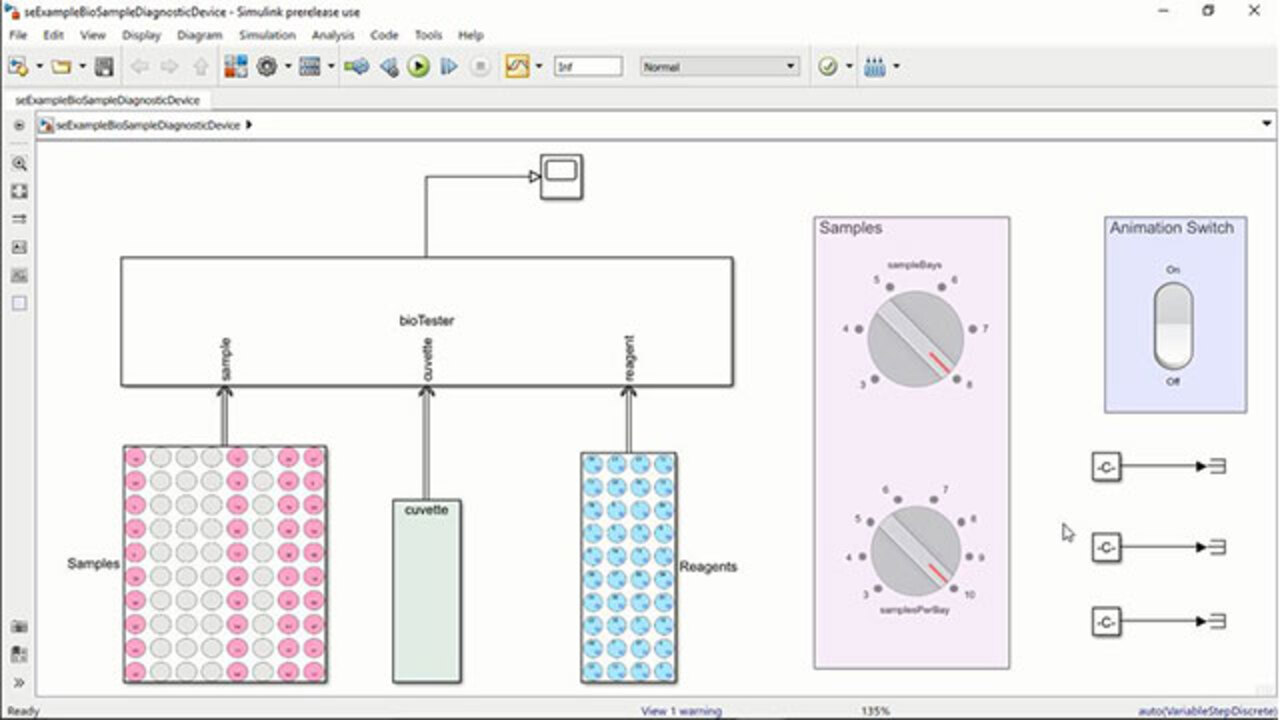
Model and Simulate In Vitro Diagnostic (IVD) Devices with SimEvents
Engineers developing complex electromechanical and fluidic devices, such as an in vitro diagnostic device, as shown in this example, tend to deal with various design and quality issues, especially around scheduling and optimizing the flow of processing samples and reagents. These issues can be addressed by having a functional simulation model of such a device before building one. View a simulation model of an in vitro diagnostic device built in SimEvents®. With such models, medical device engineers can thoroughly test their scheduling logic in simulation before implementing it in hardware and embedded software.
Published: 10 Apr 2018